I was recently reading the results of two Phase 3 psoriasis clinical trials testing a drug for use in patients with plaque psoriasis. But I don’t really want to talk about the results of these trials. I want to talk about race.
Specifically, how these trials, and many other clinical trials like them, include a disproportionate number of white people in their samples.
Prevalence of Psoriasis Globally
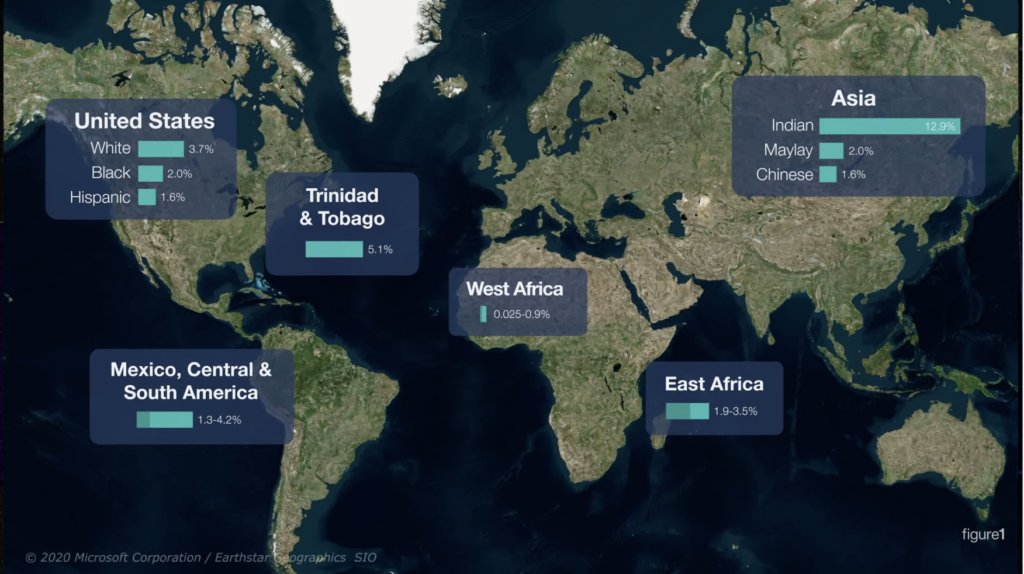
First, let’s look at the prevalence of psoriasis across the world, and in turn, across racial groups, just to get a baseline on how common this disease is.
Eastern Africa sees psoriasis rates of around 2–3.5%. Western Africa however, has rates below 1%. Why the difference? The leading theory suggests that this is because of genetic differences between these populations.
People of Asian ancestry also have wide variations in psoriasis prevalence. According to one study, 13% of people from India develop psoriasis, compared to 8.5% of people from Malaysia, and 5% of people from China. This too could be due to genetic differences, although that’s much less clear in this case.
Psoriasis rates in Mexico, Central America, and South America range between 1.5–4%. And in Trinidad and Tobago, 5% of people develop psoriasis.
When we look at the United States, just over 3.5% of white people develop psoriasis, 2% of Black people develop it, and around 1.5% of Hispanic and Latinx Americans develop it. It’s worth mentioning though that both Black and Hispanic people are likely being underrepresented in the United States data.
The reason for this is well-known. These ethnic groups have been historically treated poorly in western medicine and medical research, which has made them rightfully wary to participate in studies. That makes it harder for researchers to recruit participants from these groups, and in turn, we’re often underrepresenting Black, Indigenous, and people of color in medical research today.
Psoriasis Clinical Trial Sampling
So let’s step back and look at the larger picture. As we look at the prevalence levels of psoriasis across these populations, white people get psoriasis just about as much as other racial-ethnic groups. More or less. So when a study is testing new medication to treat psoriasis, we should see proportionate representation across all ethnicities, more or less, right?
Although it’s what we should see, it isn’t what we actually see in practice.
Let’s look at the racial demographics of the Phase 3 psoriasis clinical trials I mentioned earlier. In both trials, 85% of participants were white. Only 3–6% of participants were Black people, and only 5–9% of participants were Asian people.
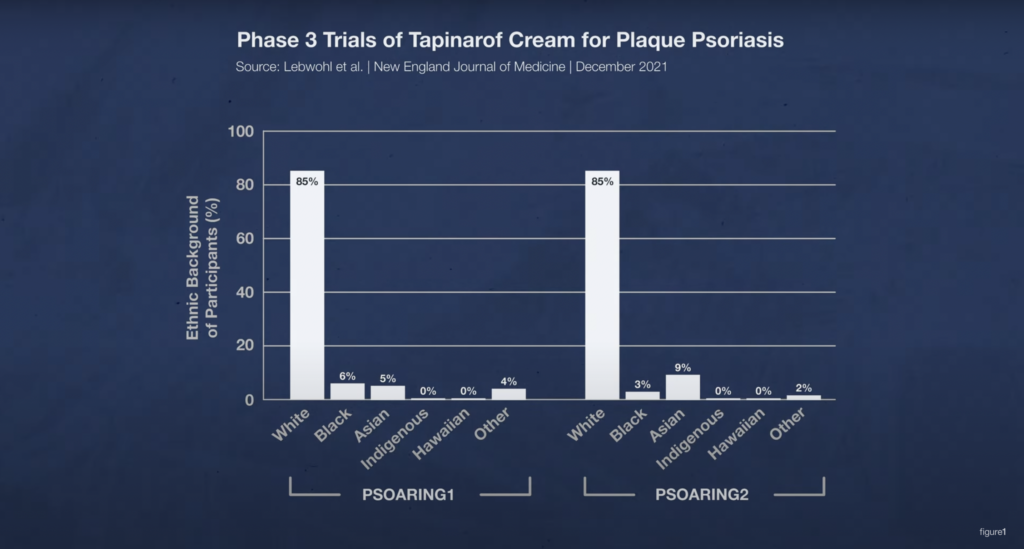
Let’s flip back to the data on the prevalence of psoriasis. In the U.S., where these clinical studies were done, white people are about twice as likely to get psoriasis as Black people. At most.
Yet in these clinical studies there are 14 to 28 times more white participants than Black participants. So how is this representative of the population of psoriasis patients?
Now, I can hear you saying, “Kyle, there’s a lot of white people in the United States and Canada. The researchers were probably matching the demographics of participants to the demographics of those countries.” And I’d say, no, they’re not, and show you census data like this: In the United States, less than 60% of people are white.
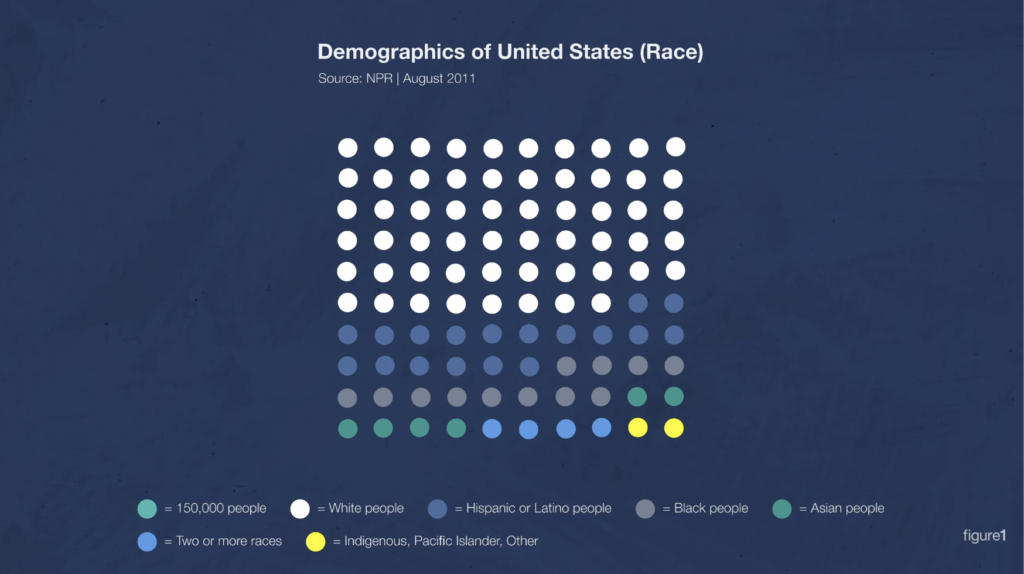
You could also say, “Hey, maybe these were just two studies that overrepresented white people in their sample.” And I’d say, hold on to your hat, because this is happening all the time.
Just look at all of these Phase 3 trials that have been done on treatments for psoriasis. And look at the percentage of white people across all those studies. This problem is prevalent!
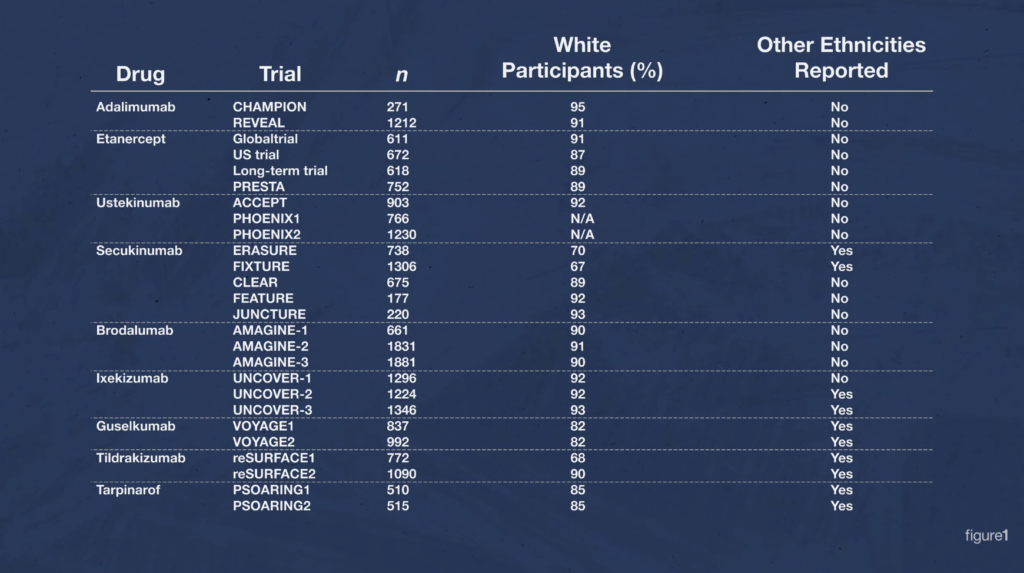
And many of the Phase 3 trials didn’t bother to break down the different ethnicities of their participants. They just referred to them as “Caucasian,” or “other.”
Why This Matters
So why does this matter? Well one, taking steps to equally represent all races as much as possible is just the right thing to do.
But two, treatments like pharmaceutical drugs don’t always affect people of different races in the same way. In fact, making this assumption could leave some groups of patients vulnerable to adverse outcomes. We simply can’t know the benefits and risks for all patients if we keep testing new treatments almost exclusively on white people.
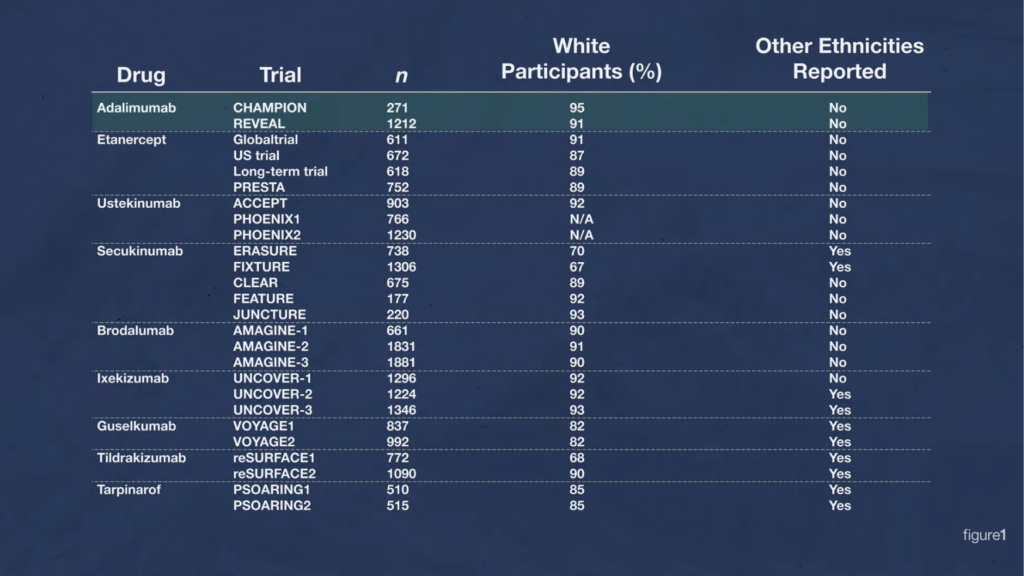
Let me illustrate what I mean. Adalimumab is a drug used to treat hidradenitis suppurativa, a disease with a global prevalence between 0.5% and 4%, just like psoriasis. I did a video on it a while back, check it out. Anyway, this drug can also be used to treat plaque psoriasis.
Here’s that chart of Phase 3 clinical trials again. There’s adalimumab. Of study participants, 91–95% were white in the two Phase 3 trials that were done for it, and the researchers didn’t provide data for any other racial-ethnic group.
It turns out that adalimumab has an interaction with people who have the HLAB*15:02 allele in the BCL2 gene. It causes Stevens-Johnson syndrome/toxic epidermal necrosis, a spectrum of very serious and very deadly skin diseases where large portions of the skin on your body blisters and peels off. This gene variant is present in 8% of Han Chinese people, an ethnic group that makes up about 18% of the global population — so, about 1.5 billion people. But this gene variant only appears in 1% of white people, or eight times less.
Representation matters in clinical trials. Without it, researchers might miss some serious drug interactions in studies that have the specific purpose of testing the safety of treatments for the general population, not just the safety of treatments for white people.
How We Can Move Forward
So, to summarize. These clinical trials, just like many other dermatology clinical trials, are for some reason heavily oversampling white participants. Their samples are not representative of the populations of the United States or Canada, nor are they representative of the larger global population, even though the prevalence of psoriasis across different ethnic groups is in the same general ballpark. The exclusion of different ethnic groups in clinical trials could and might just cause researchers to miss potentially fatal drug interactions. This practice needs to stop.
Here are a few measures that can start to help:
- Regulatory institutions like the FDA or Health Canada could put requirements in place for proportional representation.
- Grant funding for drug research and development could be tied to specific diversity targets in sample groups.
- Healthcare researchers, professional bodies, and institutions could take steps to rebuild the trust of groups that have been historically harmed through medical research.
- And healthcare researchers themselves should be aware of the risks of oversampling white participants. They too, need to take steps to make their studies more inclusive.

By Kyle Slinn, RN, BScN, MEd
Registered Nurse
Published June 6, 2022
Join the Conversation
Sign up for Figure 1 and be part of a global community of healthcare professionals gaining medical knowledge, securely sharing real patient cases, and improving outcomes.